What are pH monitoring systems and how are they applied in control systems?
This article explores the use of pH sensors in the industry, as well as the basic setup of a pH control system. A fundamental understanding of the pH scale and acid-base reactions are recommended before reading this article.
pH Monitoring and Control Applications
pH probes and transmitters are used in laboratories and control systems throughout a variety of industries including chemical production, petrochemicals, water and wastewater treatment, food and beverage, pharmaceuticals, and power plants.
A myriad of applications exist; some as novel as managing a brewery’s mash pH to produce the perfect beer, some as necessary as monitoring mining activity runoff to ensure environmental protection standards are met. Across all industries and applications, however, measurements taken from these devices are important monitoring and control parameters for product quality and process waste streams.
Probe and Transmitter Considerations
While useful, pH probes and transmitters have some practical limitations to consider.
pH meters can have a relatively slow update time, which introduces lag into the measured variable – an important consideration if pH is used for control purposes.
The exterior of many pH probes is made of glass, which makes them fragile. Other housings exist for applications where contamination due to broken glass is a concern, such as in the beverage industry.
The porous glass membrane used on most probes must stay submerged in solution to avoid drying out and causing erroneous readings. If an application will require the probe to be dry for any length of time, a specialized probe to withstand this should be considered, or the probe will have to be rehydrated in a highly ionized solution before continued use.
pH probes must also be calibrated at multiple points and cleaned quite often to ensure accurate readings and control.
What Is a pH Control System?
Achieving a target pH, either as a step in a production chain or to neutralize a waste stream as part of treatment, is a common control application.
Figure 1 shows the basic setup of a pH control process realized on an industrial scale.
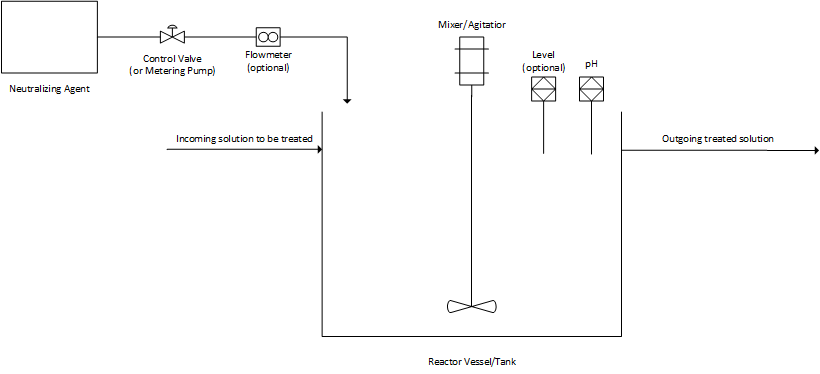
Figure 1. The basic setup of a neutralization process
Individual setups vary, but they all share some common components; namely, a reactor vessel with a mixer or agitator, chemical addition feeds that can be turned on and off in order to add the neutralizing or other target pH agent, a way for the solution requiring adjustment to enter and exit the reactor vessel, and of course, a controller such as a programmable logic controller (PLC) or distributed control system (DCS) to manage the system inputs, outputs and program actions.
The Difficulty of pH Control: Non-Linearity
Many controlled variables in the process realm behave relatively linearly. For example, opening a flow control valve some percentage causes the associated flow rate to increase. pH adjustments generally do not behave in this manner. For example, consider the titration (neutralization) of a weak acid, such as acetic acid, with a strong base, such as sodium hydroxide. The behavior of this reaction is illustrated in Figure 2, below.
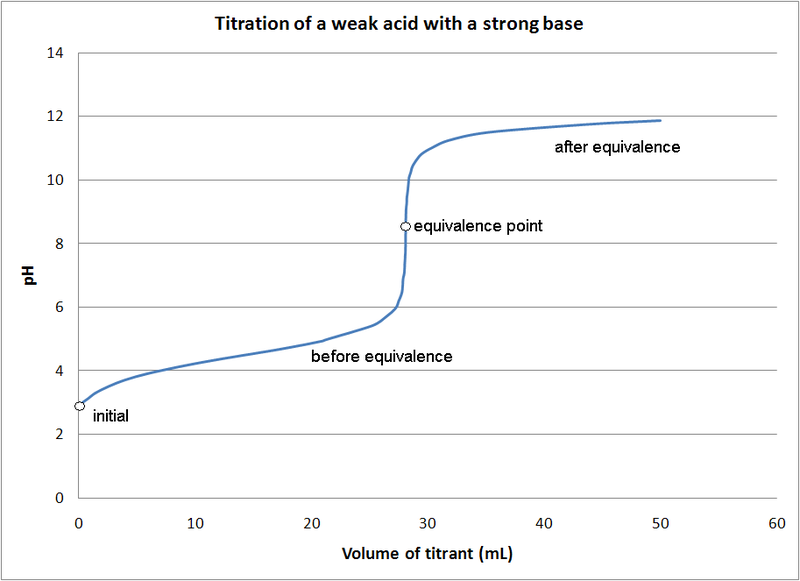
Figure 2. Titration of a weak acid with a strong base
The starting pH of the acid is around 3. When 10 mL of base are added, the pH rises by 1 pH point. When 10 more mL are added, the pH rises slightly less than 1 pH point. Then, when 10 more mL are added (30 mL total) the pH jumps drastically by over 4 pH points to 11.
This behavior is highly non-linear, rendering traditional control methodologies, such as a Proportional-Integral-Derivative (PID) loop, generally inadequate when the target pH occurs in or after the highly sloped region of the reaction. Depending on the time available to treat the influent and the variability of the influent characteristics, controls of varying complexity may be employed – anything from dosing a set amount of titrator at a specific frequency to employing more advanced predictive control algorithms.
pH Monitoring and Control: Summary
- pH monitoring and control are widely used across various industries. Selecting the proper probe and transmitter for a particular application as well as maintaining the equipment are necessary to ensure proper readings.
- pH control may be achieved with an automated control system, but the non-linear nature of pH adjustment should be considered when designing a control scheme.
Copyright Statement: The content of this website is intended for personal learning purposes only. If it infringes upon your copyright, please contact us for removal. Email: admin@eleok.com
